

Consequently, the elements in the upper right of.

In contrast, atomic size decreases from left to right and from bottom to top. Ionization energies, the magnitude of electron affinities, and electronegativities generally increase from left to right and from bottom to top. Although the valence electrons participate in shielding, electrons in the same energy level do not shield each other as effectively as the core electrons do. 1: Summary of Periodic Trends in Atomic Properties. When the attraction is weakened by shielding, the valence shell cannot be pulled closer. Sections below cover the trends in atomic radius, first ionization energy, electronegativity, melting and boiling points, and density. The smallest atom on the periodic table is helium, He, and has a radius of 31 pm. All our trends describe the trend in two directions on the periodic table: 1) across a row, and 2) up and down a column. When the nucleus pulls strongly on the valence electrons, the valence shell is pulled in closer to the nucleus. This page discusses the trends in some atomic and physical properties of the Group 1 elements - lithium, sodium, potassium, rubidium and cesium. A trend is generally 'it gets bigger' or 'it gets smaller' sort of thing. The inner energy levels are filled with electrons that serve as a shielding agent between the nucleus and the valence electrons causing the size of an atom to increase. For halogens and alkali metals as we go down the group, the principal quantum number ‘n’ increases and the valence electrons move further away from the nucleus. Down the group, the number of shells in the atom increases which increases its size. In a group, the atomic radius of the element increases from top to bottom of the group. This makes the electron closer to the nucleus which causes the atomic size to decrease. As we move from left to right in the periodic table, the positive charge on the nucleus or the effective nuclear charge increases making the attraction of the outer electron to increase. In the periodic table, the atomic radius of the element decreases from left to right in a period. I.e Van Der Waals radius > Metallic radius > Covalent radius This is because the atomic size is increasing, which. Van der Waal’s radius is measured by taking half of the internuclear separation between two atoms of the same element adjacent to each other in their solid state. The existence of these trends is due to the similarity in atomic structure of the elements in their group. Therefore, their radius is measured using Van der Waal’s method. Atomic radii can also be measured by X-ray and other spectroscopic methods.Īlternatively, the noble gases are monatomic and their non-bonded radii values are very large. Likewise, depending on whether the element is metallic or non-metallic, the atomic radius can be referred to both covalent and metallic radius. For example, the covalent radius of an element is measured by taking half of the distance between two atoms bound together by a single bond whereas a metallic radius can be calculated by taking half the internuclear distance separating the metal cores in the metallic crystal. An estimate of atomic radii can be made by knowing the distance between atoms in a combined state. Hence, the determination of atomic size cannot be precise.
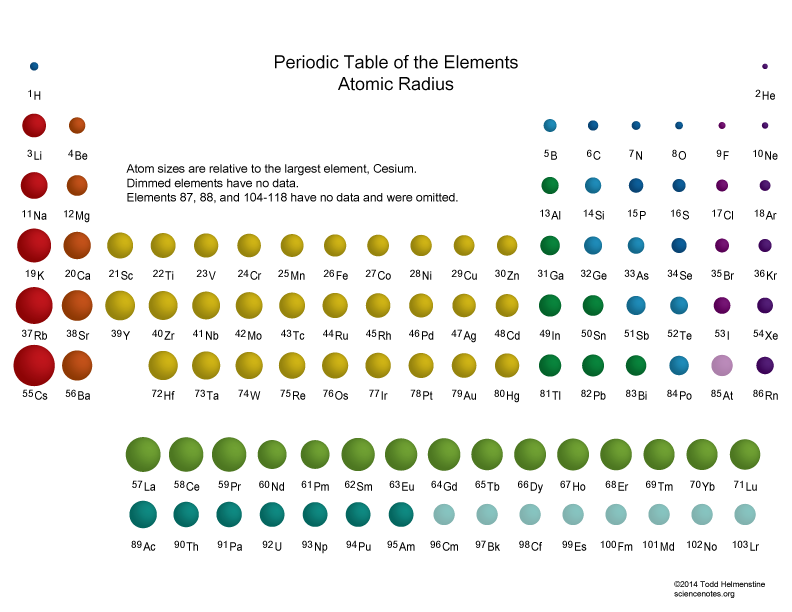
When an electron is added, a new proton is also added to the nucleus, which. Within a period of elements, each new electron is added to the same shell. The first atomic radius periodic trend is that atomic size decreases as you move left to right across a period. The pK a of the thiol group on the cysteine side chain, for example, is approximately 8.3, while the pK a for the hydroxyl on the serine side chain is on the. Atomic Radius Trend 1: Atomic Radii Decrease From Left to Right Across a Period. More importantly to the study of biological organic chemistry, this trend tells us that thiols are more acidic than alcohols. The effective charge (Zeff) of an atom is the net positive charge felt by valance. Since an electron cloud surrounds an atom, it does not have sharp boundaries. HI, with a pK a of about -9, is one the strongest acids known. periodic table, trends emerge that help explain how atomic radii changes. Chemistry LibreTexts.The atomic radius is a distance from the centre of the nucleus to the outermost shell of the electrons in the atom of any element. Periodic Trends Periodic Properties of the Elements Periodic Trends in Ionic Radii Periodic trends are specific patterns that are present in the periodic table that illustrate different aspects of a certain element, including its size and its electronic properties.


 0 kommentar(er)
0 kommentar(er)
